INTRODUCTION
India is among the top 12 destinations for biotechnology worldwide. The industry comprises around 5000 biotech companies, with 4,240 being start-ups and 760 being core biotech companies, with the number of startups expected to touch 10,000 by 2024.
India has 665 FDA-approved plants in the US; 44% of the global abbreviated new drug applications (ANDA) and more than 1400 manufacturing plants, which are compliant with WHO’s requirements.
The country is also the world’s third-largest producer of recombinant Hepatitis B vaccine and second-largest producer of BT cotton (genetically modified pest resistant plant cotton).

MARKET SIZE
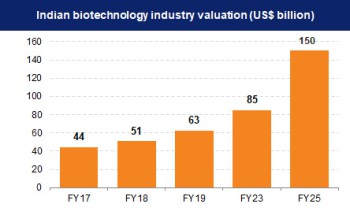
The Indian bioeconomy grew from US$ 62.5 billion in 2019 to US$ 70.2 billion in 2020 at a growth rate of 12.3%.
The Indian biotechnology industry, which stood at US$ 63 billion in 2019, is expected to reach US$ 150 billion by 2025, with a CAGR of 16.4%. By 2025, the contribution of the Indian biotechnology industry to the global biotechnology market is expected to grow to 19%. In the Indian biotechnology market, biopharmaceuticals is the largest segment, accounting for 62% share in 2020.
The Indian biologics market is forecasted to reach US$ 12 billion by 2025, at a CAGR of 22%.
As of 2021, India’s biotech industry clocks in about US$ 12 billion in annual revenue.
Bio-services, which accounted for 15% of the biotechnology industry in 2020, is becoming a leading destination for clinical trials, co
INVESTMENTS AND RECENT DEVELOPMENTS
India allows 100% FDI under the automatic route (a non-resident or Indian company will not require any approval from the government) for greenfield pharmaceuticals and manufacturing medical devices. Some recent developments/investments in the Indian pharmaceutical sector are as follows:
February 2022:
- ARISTA Biotech announced its plans to set up a cleanroom environment production facility in Hong Kong to manufacture Covid-19 rapid antigen testing kits to serve the rapidly-increasing local demand.
- Gennova Biopharmaceuticals, who were conducting phase 2 and 3 trials of India’s first indigenous mRNA vaccine on humans, have finished those trials. The data is currently being reviewed by the Drug Controller General of India (DCGI).
- American biotech company Vaxart announced plans to start phase II clinical trials of its oral tablet-based Covid-19 vaccine in India soon.
- India administered the world’s first DNA vaccine – ZYCOV-D in Patna, which was developed by Ahmedabad-based vaccine manufacturer Zydus Cadila.
- The Malaysian Drug Control Authority (DCA) approved Bharat Biotech’s Covaxin to be used in Malaysia.
- DCGA of India granted restricted emergency use authorisation to Biological E's Covid-19 vaccine Corbevax for kids in the 12-18 years age bracket.
- In January 2022, Bharat Biotech received permission from the DCGI to start trials of its intranasal booster dose in India.
In November 2021:
- Karnataka announced its aim to be a US$ 50 billion bio-economy by 2026, from the current US$ 22.6 billion.
- Serum Institute of India restarted deliveries of Covid-19 shots to global vaccine-sharing platform COVAX for the first time since April 2021.
- INOVIO announced that it has received authorisation from India's Central Drugs Standard Control Organization’s (CDSCO) Drug Controller General of India (DCGI) to proceed with the Phase 3 segment of INNOVATE (INOVIO INO-4800 Vaccine Trial for Efficacy) in India.
- The World Health Organization (WHO) issued an emergency use listing (EUL) for Bharat Biotech’s Covid-19 vaccine COVAXIN. It was found to have 78% efficacy against Covid-19 virus of any severity.
- US-based Akston Biosciences announced that it will start clinical trials of its second-generation Covid-19 vaccine ‘AKS-452’ in India.
- In October 2021, drug firm AstraZeneca launched a clinical data and insights division in Bengaluru, India, for data-related management of its clinical trials.
- In October 2021, India and Colombia exchanged views on possible scientific cooperation in the biotechnology sector. Columbia is willing to tie-up with India in the areas of vaccines, biosimilars and medical devices.
- In August 2021, Bharat Biotech received World Health Organisation’s (WHO) prequalification approval for Rotavac 5D for prevention of rotavirus diarrhea.
GOVERNMENT INITIATIVES
- February 2022: In the Union Budget 2022-23, the Department of Biotechnology was allotted Rs. 2,581 crore (US$ 343.56 million) for developing basic infrastructure, genetic engineering, technologies and bioinformatics, agriculture biotechnology, and training skilled professionals.
- In November 2021, Minister of Science & Technology Dr. Jitendra Singh inaugurated a new Biotechnology Centre for Northeast tribals in the remote area of Kimin (Arunachal Pradesh). He also launched a pan-India Star College Mentorship Programme for young innovators supported by the Department of Biotechnology.
- The Government of India and Karnataka government funded Biomoneta, a start-up that developed an air decontamination technology which eliminates airborne Covid-19 virus with 99.99% efficiency in any closed setting.
- In August 2021, the Central Council for Research in Siddha (CCRS) introduced ARIVU, an initiative to motivate academics and the industry to carry out research to advance value-chain in industries such as biotechnology and nanotechnology.
Department of Biotechnology:
- Atal Jai Anusandhan Biotech Mission was implemented by Department of Biotechnology (DBT), Ministry of Science and Technology. The purpose of this mission is to address the challenges of maternal and child health, antimicrobial resistance, vaccines for infectious disease, food and nutrition, and clean technologies.
- In October 2021, the Department of Biotechnology launched a ‘One Health’ consortium that will survey important bacterial, viral and parasitic infections of zoonotic as well as transboundary pathogens in the country. The consortium consists of 27 organisations and is led by the DBT-National Institute of Animal Biotechnology, Hyderabad.
BIRAC
- Biotechnology Industry Research Assistance Council (BIRAC) established by the Department of Biotechnology (DBT) is aimed at strengthening and empowering emerging biotechnology enterprises to undertake strategic research and innovation.
- In February 2022, BIRAC invited proposals for development, validation and pre- commercialization of products/ technologies in the areas of healthcare, energy and environment, veterinary sciences and aquaculture, agriculture and secondary agriculture.
- In October 2021, Jawaharlal Institute of Postgraduate Medical Education and Research in Pondicherry established a research unit funded by BIRAC to undertake clinical trials of new Covid-19 vaccines that are currently in their development stage.
- In October 2021, BioNEST Bioincubator, a healthcare innovation incubation centre, was inaugurated at the Sri Ramachandra Institute of Higher Education and Research in Chennai, Tamil Nadu.
- In July 2021, HempStreet became the first medicinal cannabis firm to win a BIRAC grant.
- In July 2021, DBT-BIRAC funded SanMitra 1000 HCT, a hand-cranked dual powered (grid+hand cranked) defibrillator developed by Jeevtronics Pvt. Ltd. Experts believe that this low-cost, light-weight device was more reliable than standard defibrillators as it can be used in regions where electricity is absent.
Biotech Parks:
- Biotechnology Parks and Incubators are established across the country by the Department of Biotechnology (DBT), under the Ministry of Science and Technology, to translate research into products and services by providing the necessary infrastructure support.
- These biotechnology parks offer facilities to scientists, and SMEs for technology incubation, technology demonstration and pilot plant studies to accelerate the commercial development of biotechnology.
- The government supports nine biotechnology parks in various states with the bulk being in the southern region of the country.
ROAD AHEAD
Indian biotechnology is built on entrepreneurship, innovation, developing domestic talent and demonstrating value-based care.
Given the long history of diseases in India, the country has accumulated years of experience and scientific knowledge to prevent and treat them. India is working to boost the biotechnology sector under various flagship programmes such as 'Make in India' and 'Start-up India'.
Increase in the number of biotech incubators will boost research and promote growth of start-ups, which is critical for the success of the Indian biotech industry.

References:Department of Biotechnology, Association of Biotechnology Led Enterprises, BIRAC, Global Bio-India
Note:Conversion rate used in November 2021, Rs. 1 = US$ 0.01336
Disclaimer:This information has been collected through secondary research and IBEF is not responsible for any errors in the same
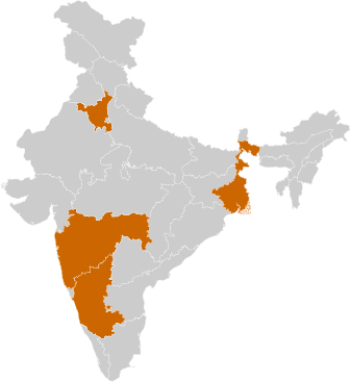
BIOTECHNOLOGY CLUSTERS
- Haryana
- Bengaluru
- West Bengal
- Maharashtra
Industry Contacts
- Department of Biotechnology, Ministry of Science & Technology
- Department of Science and Technology, Ministry of Science and Technology
- Biotechnology Industry Research Assistance Council (BIRAC)
- Council of Scientific and Industrial Research (CSIR)
- Association of Biotechnology Led Enterprises (ABLE)
- The Biotech Research Society, India